Recent Advances in Digestive System Health
Inflammatory Bowel Diseases - Crohn’s Disease and Ulcerative Colitis
In inflammatory bowel disease (IBD), the immune system overreacts and causes long-lasting inflammation in the gut. Biologic medicines are special treatments made from proteins that act like targeted blockers. Instead of calming the whole immune system, they focus on the specific signals that drive gut inflammation. By switching off these ‘bad signals,’ biologics reduce swelling, pain, and flare-ups, helping the bowel heal and improving day-to-day life.
-
IL-23 inhibitors such as guselkumab (Tremfya, Johnson and Johnson), mirikizumab (Omvoh, Eli Lilly) and risankizumab (Skyrizi, AbbVie), and JAK inhibitor upadacitinib (Rinvoq, AbbVie) have been recommended by NICE for the treatment of moderately to severely active Crohn’s disease.
IL-23 inhibitors such as guselkumab (Tremfya, Johnson and Johnson), mirikizumab (Omvoh, Eli Lilly) and risankizumab (Skyrizi, AbbVie), JAK inhibitors upadacitinib (Rinvoq, AbbVie) and filgotinib (Jyseleca, Alfasigma S.p.A.) and S1P modulator s ozanimod (Zeposia, Bristol Myers Squibb) and etrasimod (Velsipity, Pfizer) been recommended by NICE for the treatment of moderately to severely active ulcerative colitis.
-
The following home faecal calprotectin tests for monitoring treatment response in inflammatory bowel disease have been appraised by NICE:
ProciseDx (BHR Pharmaceuticals) was appraised by NICE in 2022 and is the platform for monitoring levels of inflammation and measuring levels of the therapeutic drugs infliximab and adalimumab.
-
Phase 3 UK trials are currently ongoing to assess the safety and effectiveness of tulisokibart developed by Merck and afimkibart developed by Roche in the treatment of moderately to severely active Crohn’s disease.
For ulcerative colitis, trials involving tulisokibart developed by Merck and afimkibart developed by Roche are ongoing.
-
Pioglitazone or vitamin E may be recommended for adults with advanced liver fibrosis although unlicensed for this particular indication.
Obeticholic acid and semaglutide are currently awaiting appraisals by NICE to be used in the treatment of metabolic dysfunction-associated steatohepatitis with liver fibrosis.
-
FibroScan - a non-invasive medical device that assesses liver fibrosis and cirrhosis by measuring the degree of liver stiffness - and LIVERFASt (Fibronostics) - a blood test for detecting and staging the fibrosis (scarring), activity (inflammation) and steatosis (fat build-up) of the liver - have been appraised by NICE.
-
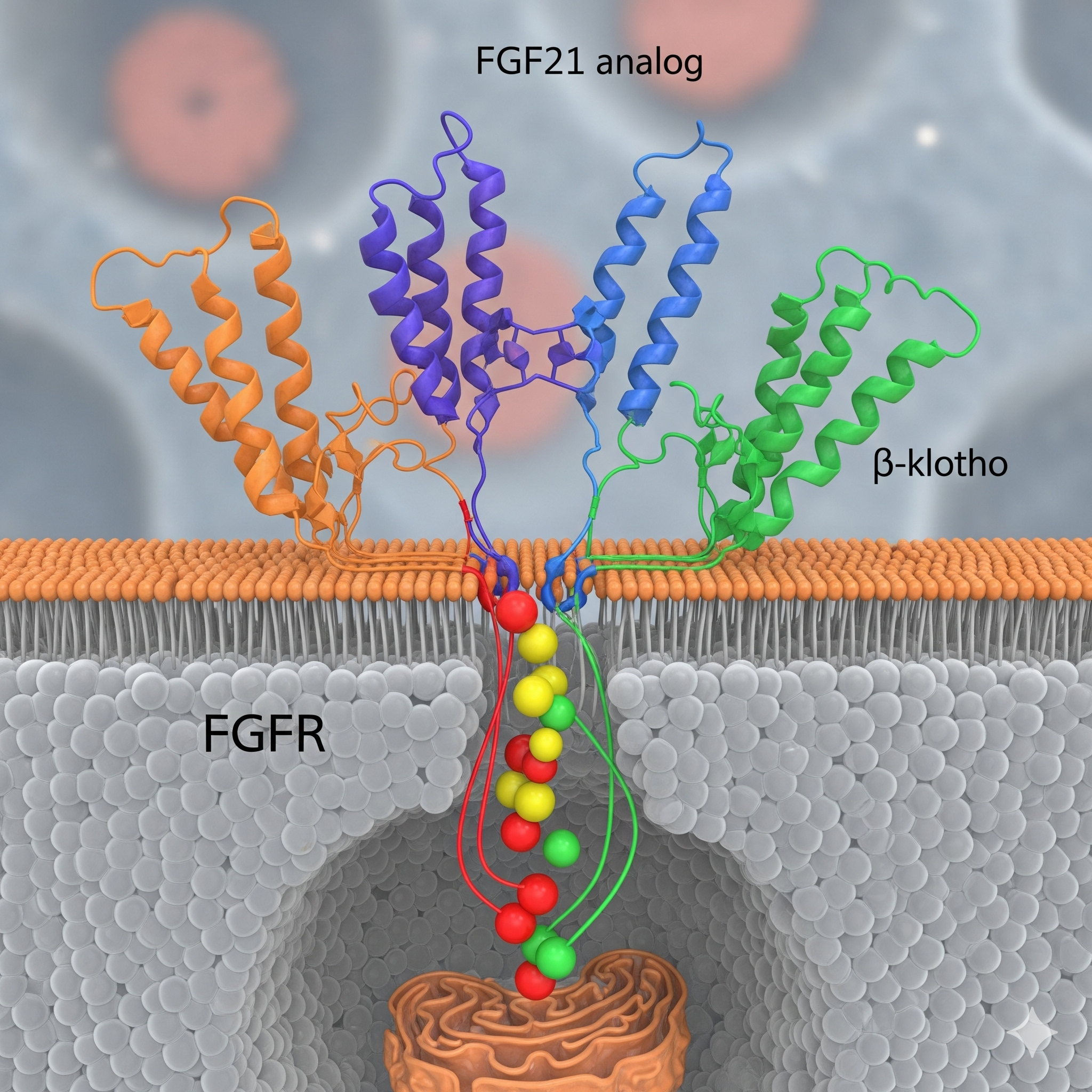
Phase 3 UK trials are currently ongoing to assess the safety and effectiveness of fibroblast growth factor 21 (FGF21) analog efruxifermin developed by Akero Therapeutics, Inc and dual agonist activating both the glucagon receptor and the GLP-1 receptor survodutide by Boehringer Ingelheim in the treatment of Metabolic Dysfunction Associated Steatohepatitis (MASH) and liver fibrosis.
Fatty Liver Disease (non-alcoholic or metabolic)
Non-alcoholic fatty liver disease (NAFLD) is fat buildup in the liver unrelated to alcohol or other causes like medications, hepatitis C, or endocrine disorders. It ranges from simple steatosis to nonalcoholic steatohepatitis (NASH)/metabolic dysfunction-associated steatohepatitis (MASH), fibrosis, and cirrhosis. NAFLD affects 20–30% of people, with 2–3% having NASH, especially those with type 2 diabetes or metabolic syndrome.
Since the late 2010s, therapies like GLP-1 receptor agonists (e.g., semaglutide) and FXR agonists (e.g., obeticholic acid) have shown potential to be repurposed in reducing liver fat, inflammation, and scarring.