-
-
A phase 3 trial with remternetug by Eli Lilly is now recruiting. Trontinemab by Roche/Genentech has finished a successful phase 2 trial.
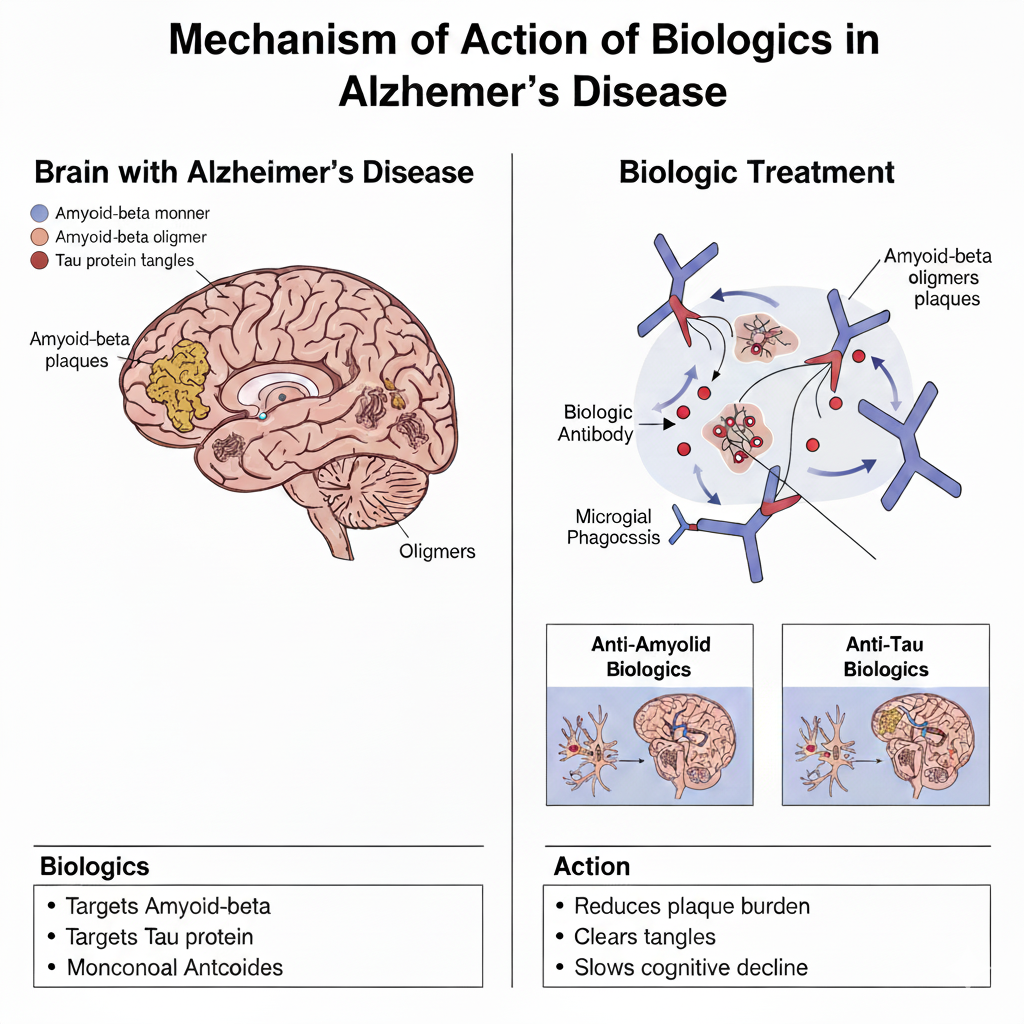
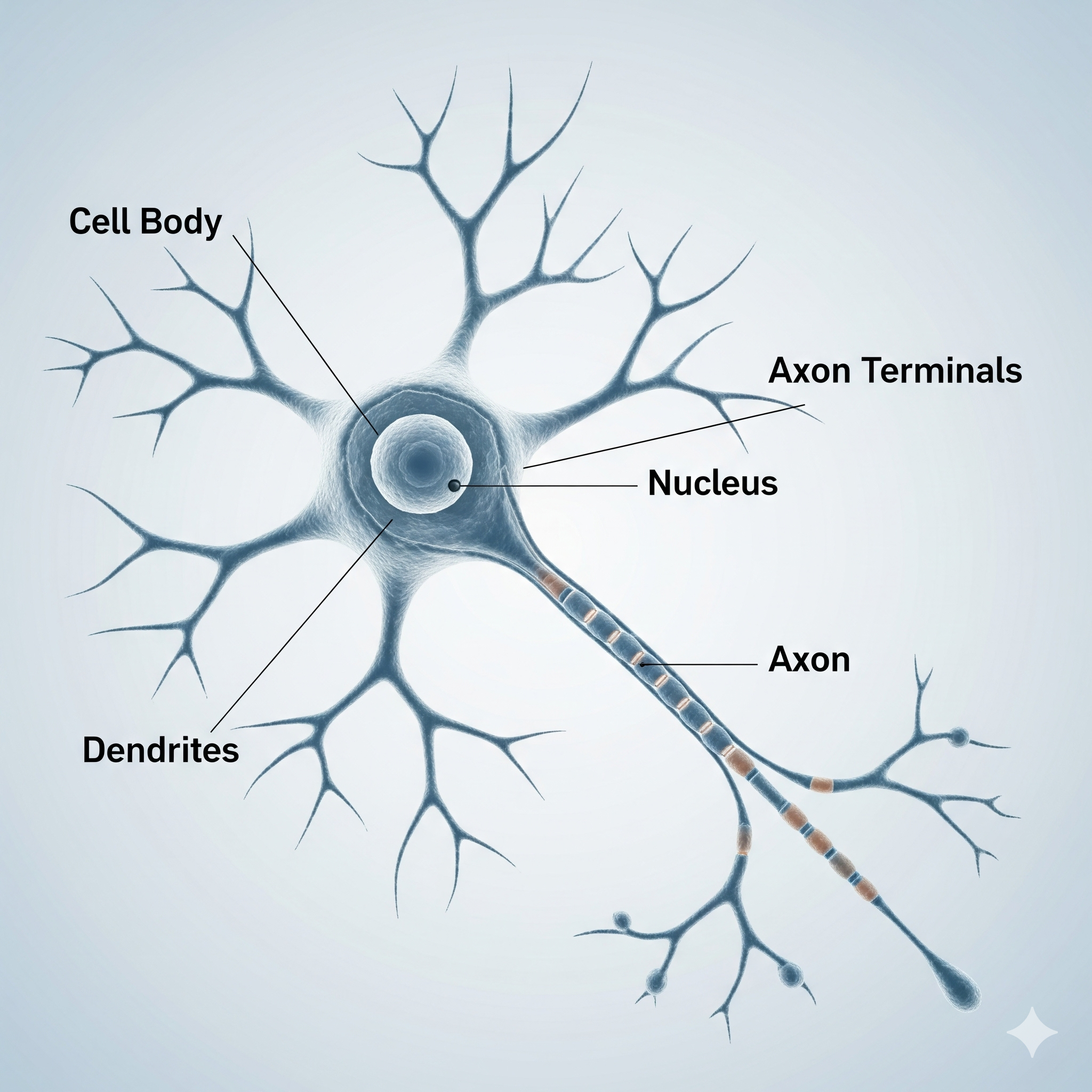
Biologics in treatment of Alzheimer’s disease
In Alzheimer’s, abnormal proteins (like amyloid plaques and tau tangles) build up in the brain. These proteins damage brain cells and affect memory and thinking.
Biologic medicines are special antibody-based treatments that target these harmful proteins:
Some biologics, like lecanemab and donanemab, bind to amyloid plaques and help the body’s immune system clear them away.
Others under development aim to prevent new plaques from forming or reduce tau tangles.
By removing or slowing the build-up of these proteins, biologics aim to protect brain cells and slow memory loss.
They don’t cure Alzheimer’s, but they may delay its progression and help people maintain independence for longer.
Wearable drug delivery device for advanced Parkinson’s disease
Produodopa developed by AbbVie is a treatment for advanced Parkinson’s disease.
How it works:
It delivers a combination of levodopa and carbidopa — the main medicines used to control Parkinson’s symptoms.
Instead of taking tablets, Produodopa is given as a continuous infusion under the skin using a small, portable pump.
This keeps a steady level of medication in the blood throughout the day.
Why this helps:
Reduces “off” periods when symptoms return between tablet doses
Provides more consistent symptom control
Improves daily activities and quality of life in advanced stages
-
Prasinezumab developed by Roche will be advancing into phase 3 development.
Biologics in treatment of Parkinson’s disease
In Parkinson’s, a protein called alpha-synuclein can build up and form clumps in the brain.
These clumps damage brain cells that produce dopamine, a chemical needed for smooth movement.
Biologic medicines are antibody-based treatments designed to:
Recognise and bind to harmful alpha-synuclein proteins
Help the body’s immune system clear these proteins
Slow down the damage to brain cells
The goal is to slow disease progression rather than just manage symptoms.
-
Kinesia360
KinesiaU
PDMonitor
Personal KinetiGraph (PKG)
STAT-ON
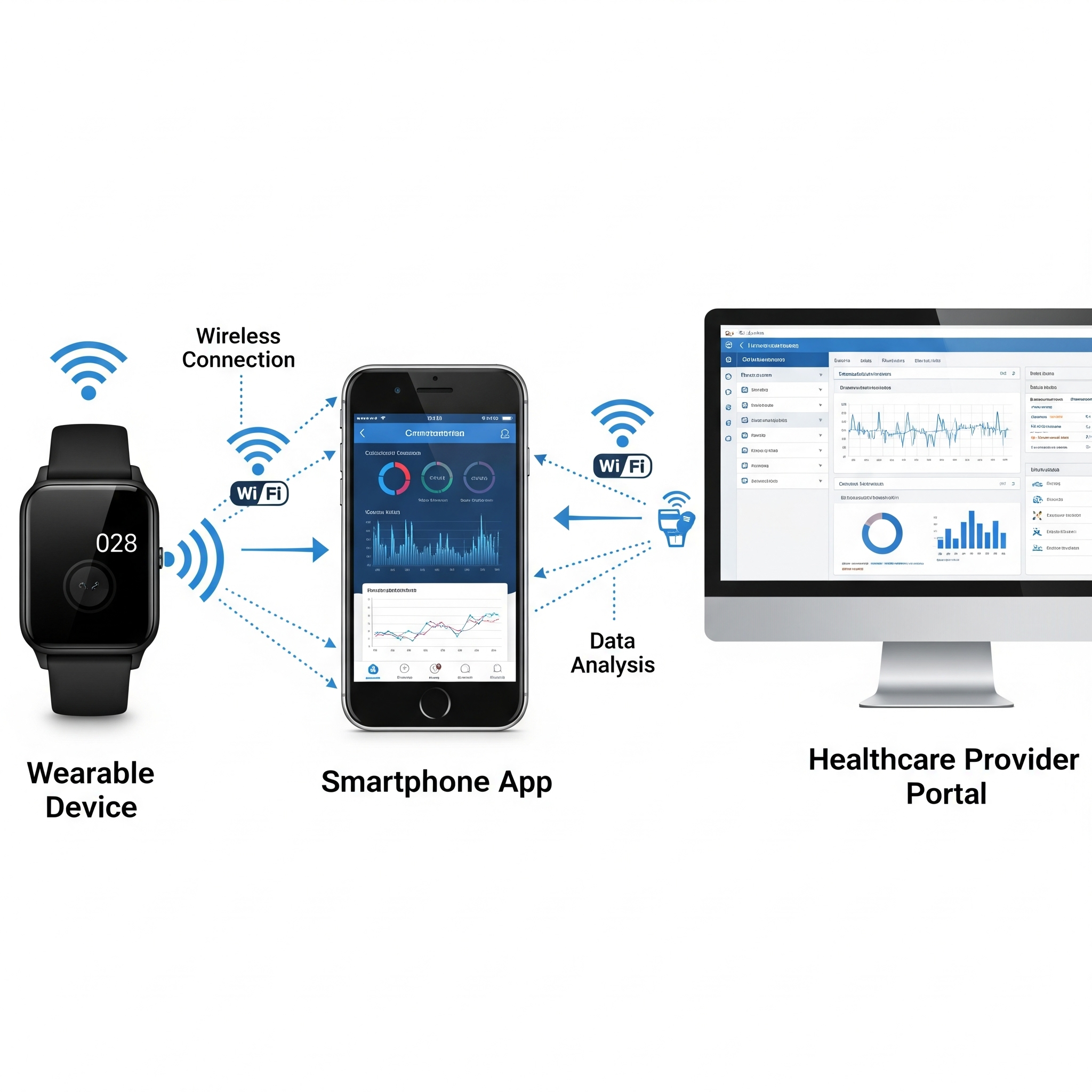
Remote monitoring devices in Parkinson’s disease
Remote monitoring devices track movement, symptoms, and treatment response at home, giving doctors a clearer view of Parkinson’s impact for personalized care.
How They Work
Wearables & smartwatches monitor tremor, movement, balance, and freezing.
Sensors & apps track activity, medication timing, and symptom changes.
Smart pens & keyboards detect subtle handwriting or typing changes.
Data is securely sent to care teams for early intervention.
Benefits
Improved symptom tracking between visits
Early detection of worsening symptoms
Personalised treatment plans
Fewer hospital trips for convenience
-
Path Finder laser shoe attachment was approved by NICE in 2019.
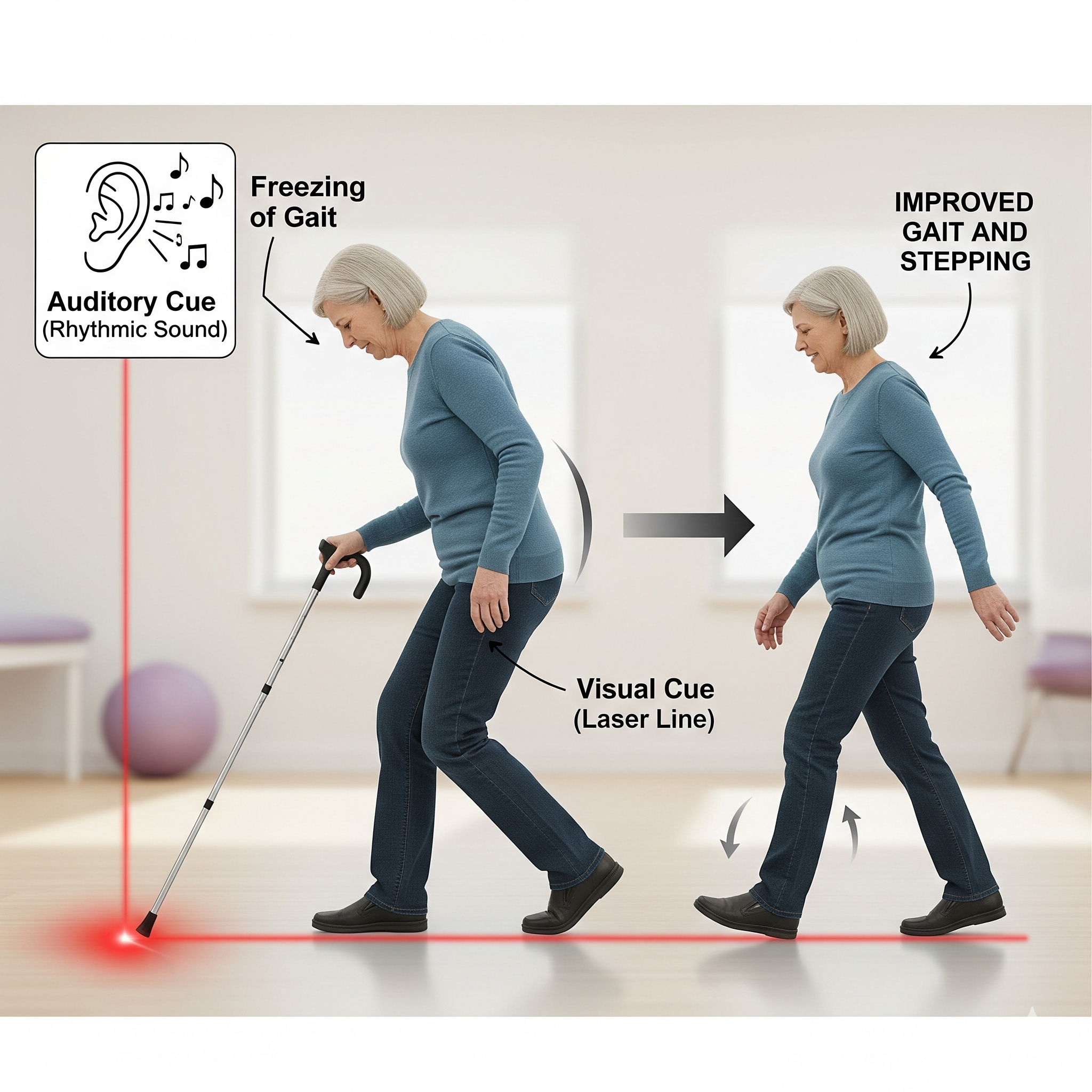
Cueing devices for freezing of gait with Parkinson’s disease
People with Parkinson’s often face freezing of gait—feet feel “stuck,” making walking difficult. Cueing devices give external prompts to help initiate and regulate movement.
How They Work
Visual: Laser lines or patterns guide steps.
Auditory: Rhythmic sounds, metronomes, or music set pace.
Vibrational: Wearables send gentle pulses to signal stepping.
These cues bypass impaired brain control, easing walking start, maintenance, and stability.
Benefits
Fewer freezing episodes
Improved walking speed and balance
Increased confidence and independence
Supports rehabilitation and home mobility
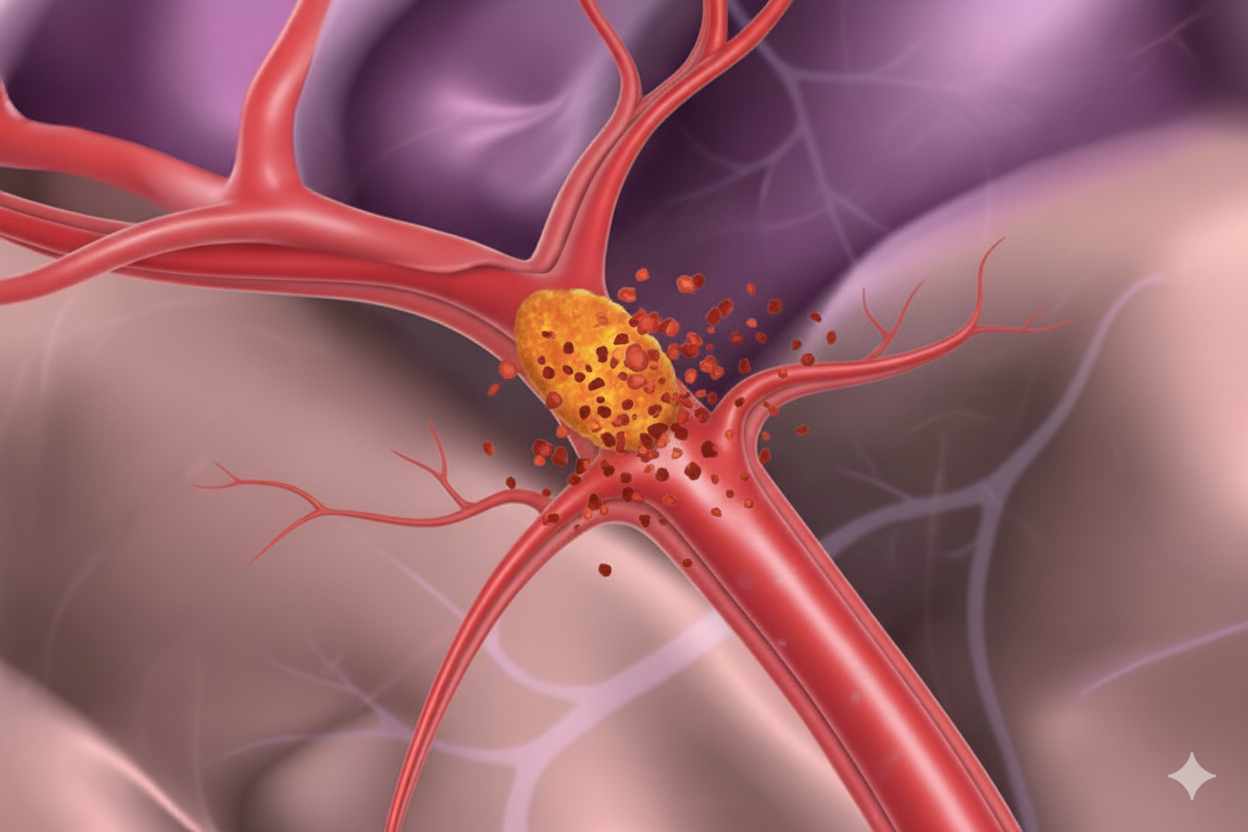
Ischaemic stroke (due to a blocked artery)
Tissue plasminogen activators (tPAs) are clot-busting drugs used in ischaemic stroke.
Tenecteplase is a newer drug showing similar or better effectiveness than the previously available product alteplase (tPA). It is easier to give — single injection instead of an hour-long drip and gaining ground in global stroke guidelines.
-
Tenecteplase (Metalyse), developed by Boehringer Ingelheim, has been approved by NICE for the thrombolytic treatment of an acute ischaemic stroke in adults:
within 4.5 hours of the onset of stroke symptoms, and
when intracranial haemorrhage has been excluded.
-
A phase 3 UK trial is testing if Milvexian (factor XIa inhibitor) reduces recurrent ischaemic stroke risk versus placebo.
Stroke rehabilitation and spasticity
Stroke rehab is evolving with robotic exoskeletons to support walking, devices to retrain swallowing and facial muscles, and virtual reality therapy to make recovery more interactive and effective.
-
ReStore Soft Exo-Suit to help with mobility and Iqoro to help with swallowing have been appraised by NICE. Mollii suit has also been appraised for treating spasticity.
Epilepsy
New anti-seizure medications with dual mechanism (through enhancing neurological inhibition and reducing excitation) are showing significant promise in the treatment of drug-resistant focal epilepsy. Additionally, subcutaneous EEG technology allows for effective long-term monitoring of patients, improving the management and understanding of this condition.
-
Cenobamate (Ontozry, Arvelle Therapeutics), anti-seizure medication with dual action, is recommended by NICE as an add-on therapy and 24/7 EEG SubQ was appraised by NICE in 2021.
-
Migraines
One of the new migraine treatments is Calcitonin Gene Related Peptide (CGRP)-blockers for both acute relief and prevention. During a migraine, CGRP levels rise, causing blood vessels in the brain to widen (dilate) and nerves to become more sensitive, leading to pain and other symptoms.
CGRP blockers (also called CGRP inhibitors) stop CGRP from attaching to its receptor, either by binding directly to CGRP or to its receptor. This prevents the chain reaction of vessel widening and nerve over-activation that drives migraine symptoms.
They can be used as preventive treatments (regular injections or tablets to reduce migraine frequency) or acute treatments (taken during a migraine attack to stop it from progressing).
-
A few CGRP-blockers have been recommended by NICE with restrictions - oral tablets such as rimegepant (Vydura) and atogepant (Aquipta), intravenous infusions such as eptinezumab (VYEPTI), and self-injected medications such as erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality).
-
Atogepant (Aquipta) developed by AbbVie, already approved by NICE for preventing migraine (currently awaiting appraisal for treating migraine), is being tested in preventing ‘menstrual migraine’ in particular in phase 3 clinical trials.